Cellgard Cell Retention Pad
2023-11-28
MS
101
With the rapid development of the domestic biopharmaceutical industry, cell perfusion culture has attracted more and more attention because it can achieve high cell density in small volume bioreactors. Among them, cell retention devices are crucial for the development of perfusion culture processes. In this article, Xiaomai will briefly introduce the disposable consumables used in cell perfusion culture and the latest product Cellgard cell retention pad developed by Membrane Solutions.
About Single-use Bioreactor
In 1998, Wave Biotech launched Wave Bioreactor® wave bioreactor system, which uses single-use bioreactor Cellbag® as a cell and microbial culture container, see Figure 1. After years of market validation, the single-use wave bioreactor system and its accompanying consumables have been widely accepted and recognized globally. With the rapid development of the life sciences and biopharmaceutical industries, industry entities have increasingly high requirements for cell culture, such as higher cell culture density, shorter culture cycles, lower culture costs, and higher success rates. Therefore, disciplines such as the preparation technology of single-use bioreactor consumables and biological culture processes are also constantly developing and updating iteratively.
 Figure 1: Single-use Bioreactor Cellbag ® Schematic Diagram (Image from GE)
Figure 1: Single-use Bioreactor Cellbag ® Schematic Diagram (Image from GE)
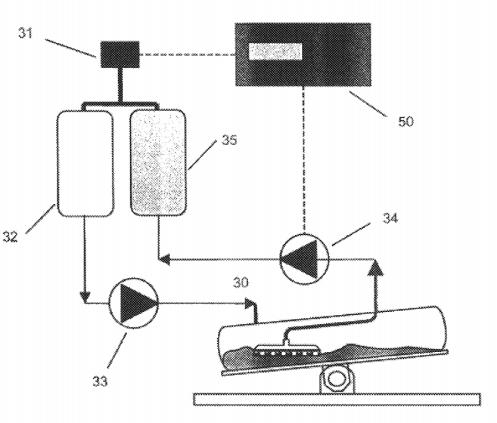
In 2001, Dr Vijay Singh invented a single-use bioreactor product with a filter, as shown in Figure 2 (patent number US6544788). This product can retention target substances such as cells, microorganisms, etc. inside the reactor through the "filter", and discharge waste culture medium liquid outside the reactor through the "filter", achieving the addition of fresh culture medium, thus achieving the continuous perfusion culture of cells and microorganisms in Wave Bioreactor® and single-use reactor products. It provides a powerful tool for microbial culture process and biopharmaceutical upstream cell expansion process. The most well-known product in the industry today is the XuriTM series single-use perfusion bioreactor product sold by Cytiva Company, as shown in Figure 3. With the expiration of patent US6544788, global manufacturers of disposable consumables have also begun the development and commercialization of single-use perfusion bioreactor.
 |
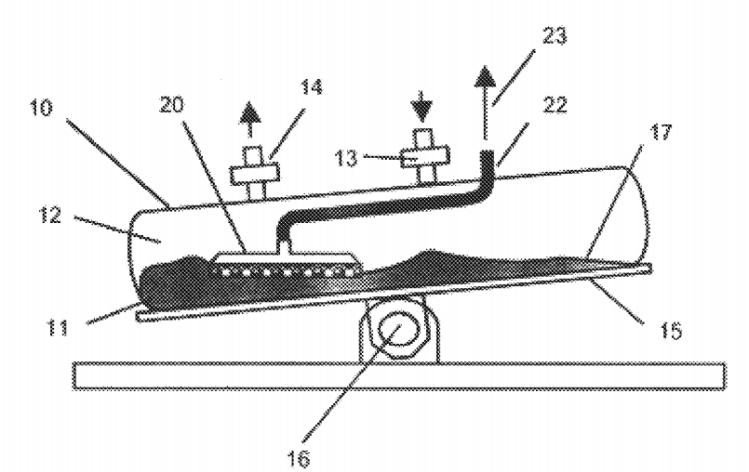 |
 Figure 3: XuriTM Series Single-use Perfusion Bioreactor Products (Image from Cytiva) Figure 3: XuriTM Series Single-use Perfusion Bioreactor Products (Image from Cytiva) |
Figure 2: Schematic Diagram of a Single-use Bioreactor with a Filter (Image source: US6544788)
The basic requirements of a single-use perfusion bioreactor is to ensure that the cultivated microorganisms or cells achieve the expected proliferation density under the biological culture process conditions of the product user, and in the process, the "filter" that plays a retention role in the process is not blocked, that is, the liquid can pass through the filtration device at a lower pressure, and the "filter" can effectively retention the cultured substance, that is, there is almost no microorganism or cell penetrating downstream of the filtration device. Therefore, as can be seen from the above, the retention performance of the "filter" is the key core component that determines whether the single-use perfusion bioreactor can meet the usage requirements.
Application and Challenge of Single-use Perfusion Bioreactor
An important application point of single-use perfusion bioreactor is located in the cell expansion process section of the biopharmaceutical process. During the cell expansion stage, users expect to achieve high-density cell culture of engineering cells such as CHO through the perfusion culture process. The benefits of high-density CHO cell perfusion culture are as follows: on the one hand, it can improve the efficiency of cell expansion, and may even reduce 1-2 cell expansion process steps, saving time, raw materials, money, and labor costs, reducing the risk of infection, and improving the success rate; on the other hand, in some small-scale research and production activities that utilize engineering cells to express target proteins, harvest viral vectors, and obtain mRNA vaccines, high cell culture density can bring higher target titers and improve efficiency. Figure 4 below compares an example of using conventional methods to expand cells to a 500L production bioreactor (n). The use of high-density cell bank method reduces the steps for cultivating the middle two rotating bottles and can be directly inoculated into a 2L wave bioreactor. The use of wave bioreactors (2L and 20L) instead of rotating bottles (125ml to 10L) greatly reduces the number of operations required in the ultra clean table. This replacement enables the entire process to be carried out in a closed system, improving the success rate of the operation.
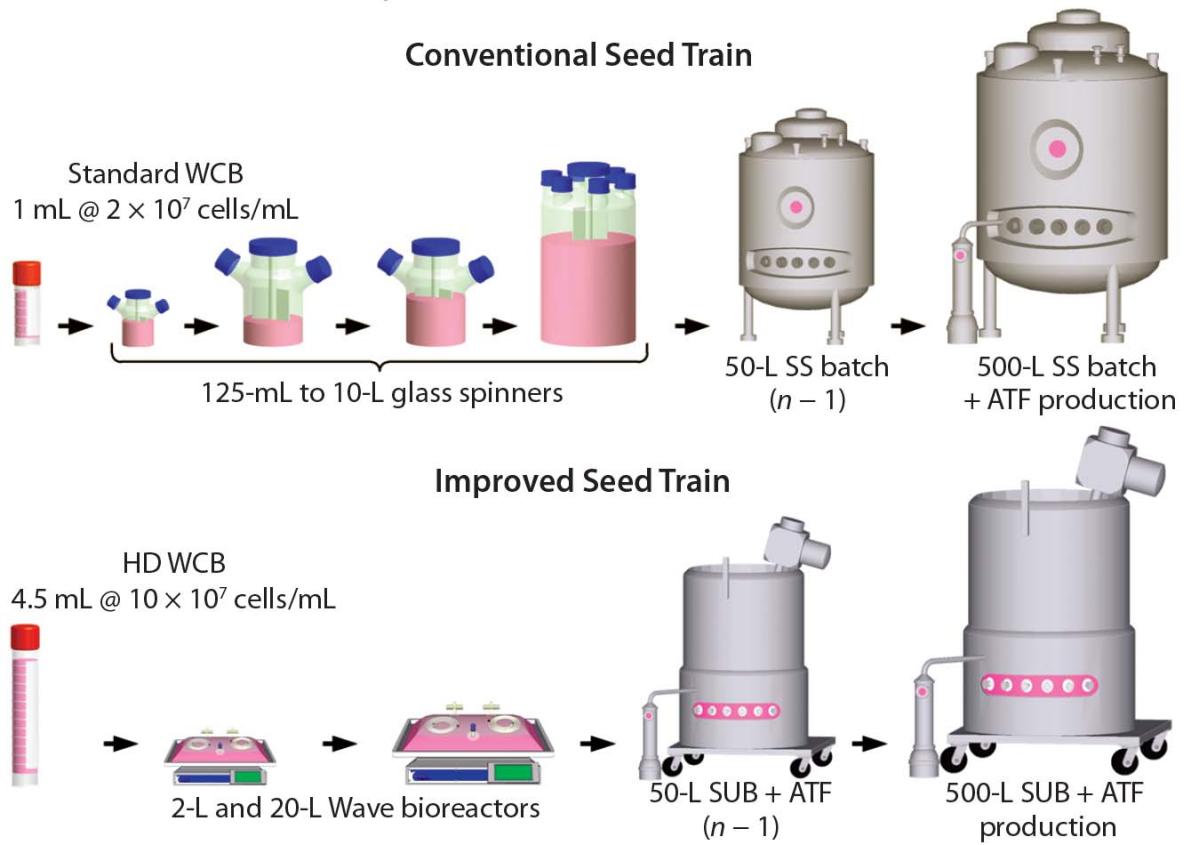 Figure 4: Comparison of Conventional and Improved Cell Culture Processes (Image Source: Bioprocess Int)
Figure 4: Comparison of Conventional and Improved Cell Culture Processes (Image Source: Bioprocess Int)
However, the challenge of high cell density for perfusion bioreactor is enormous, especially when the cell density increases, the pressure on the filtration components is particularly significant. When the cell concentration is high, the filtration component needs to be able to maintain the perfusion process, which requires the filtration component not to be blocked when facing the challenge of high concentration liquid; furthermore, the cultured material, such as cells and microorganisms, is easily penetrated by the filter medium under low pressure due to their soft structure. Another point that is easily overlooked is that the filtration components in the perfusion bioreactor are constantly "floating" with the Wave Bioreactor® during the entire culture process, and the long-term cultivation demand have put forward higher requirements for the mechanical reliability of filtration components. In summary, a qualified retention filtration component for Wave Bioreactor® needs to meet the requirements of high load, long life, high retention efficiency, and high mechanical reliability. Based on years of experience in the filtration, separation, and purification industries, Membrane Solutions proposed the use of depth filtration mechanisms to address the challenges posed by the filtration components of perfusion bioreactors, and successfully developed Cellgard cell retention pad.
About Cellgard Cell Retention Pad
The Cellgard cell retention pad developed by Membrane Solutions is a kind of depth filtration medium made by the sintering process of ultra-high molecular weight polyethylene powder, as shown in Figure 5. There are two sizes of 17×17cm and 50×30cm, and the thickness is 3mm, as shown in Figure 6. In response to the specific needs of the biopharmaceutical industry for the expansion and culture of engineering cells (such as CHO DG44 cells, CHO K1 cells, HEK 293 cells, etc.), the pore size of Cellgard is designed to be 7μm, and the puncture strength of Cellgard is up to 300N/mm, which greatly improves the mechanical reliability of the retention parts. Such a design can well meet the challenges posed by the perfusion bioreactor to the retention filtration components. In the perfusion cell culture evaluation experiments of MS and customers, the Cellgard cell retention pad perfectly achieved cell culture under different conditions and met the test expectations.
 Figure 5: MS Cellgard Cell Retention Pad Figure 5: MS Cellgard Cell Retention Pad |
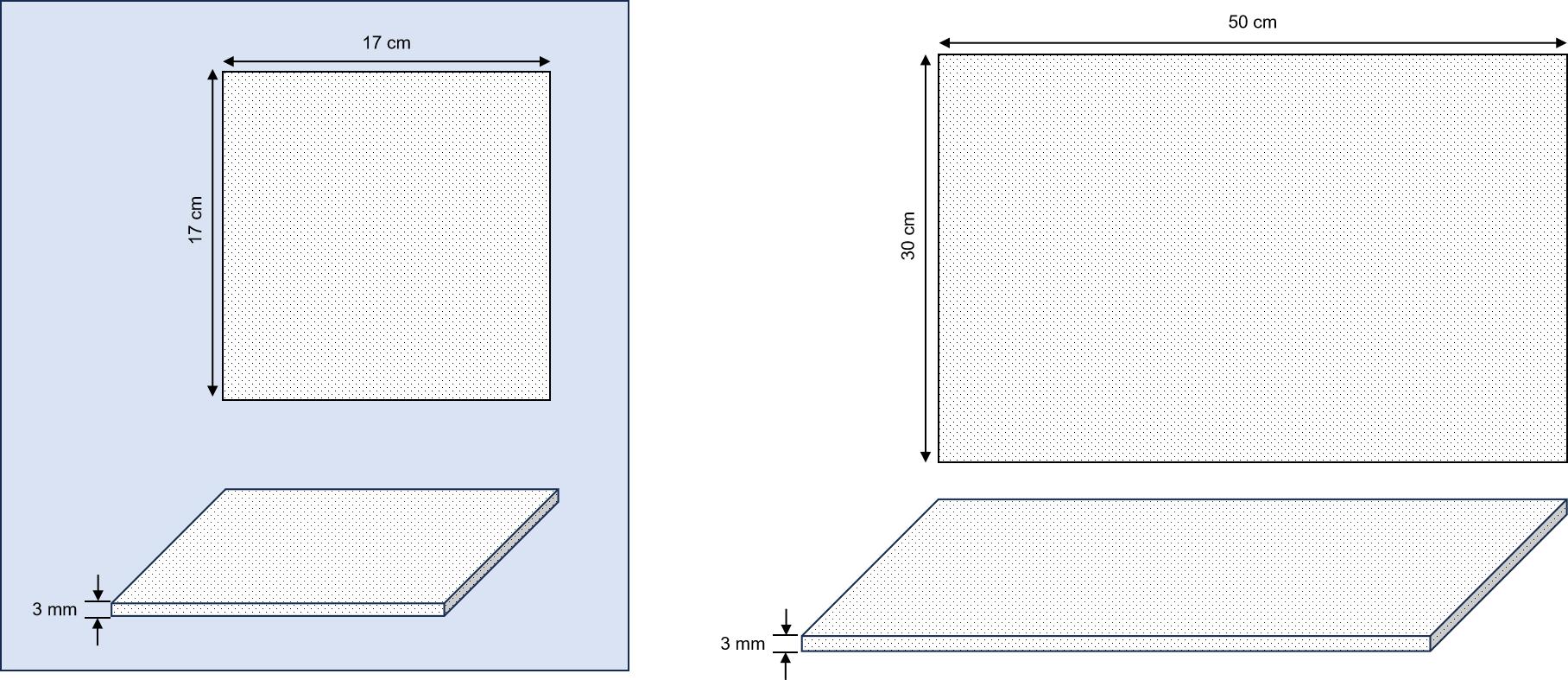 Figure 6: Schematic Diagram of the Size of MS Cellgard Cell Retention Pad Figure 6: Schematic Diagram of the Size of MS Cellgard Cell Retention Pad |
Experimental Analysis of Cellgard Cell Retention Pad Performance
Experiment 1: 2L Wave Perfusion
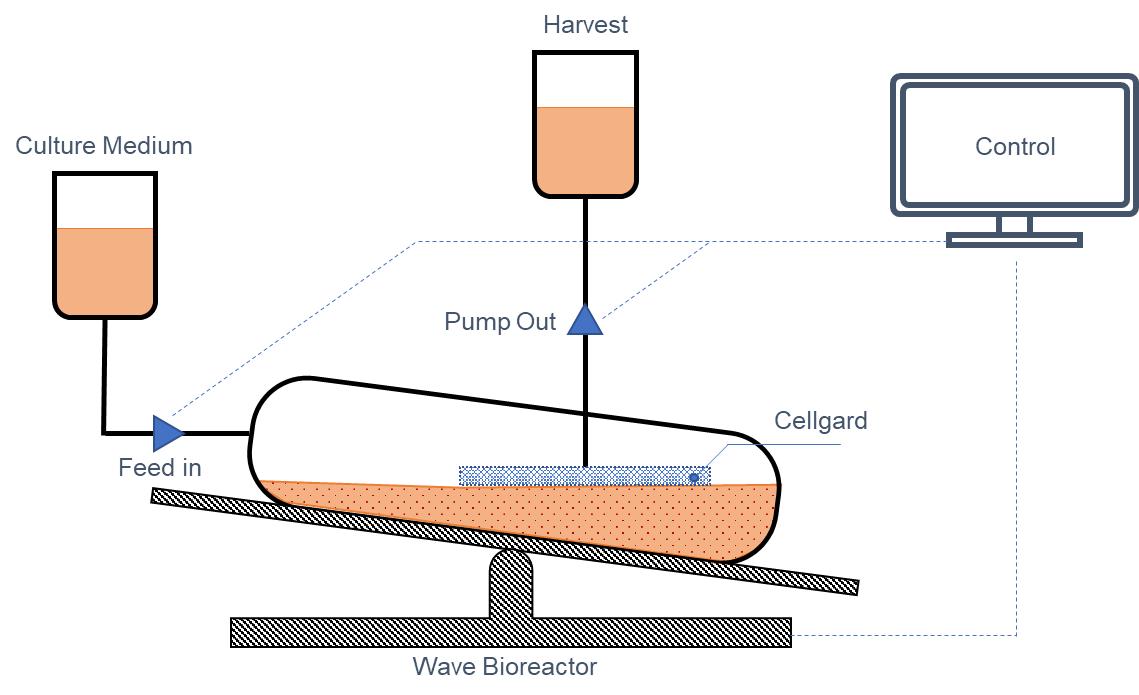 Figure 7: Schematic Diagram of the Structure of a Perfusion Bioreactor
Figure 7: Schematic Diagram of the Structure of a Perfusion Bioreactor
In the first set of tests, Cellgard cell retention pad measuring 17×17cm were fabricated into 2L perfusion bioreactor (see Diagram 7 above). This test was conducted to investigate the cell expansion of the sample perfusion bag in the absence of protein expression, using the cell line CHO K1. In the other two sets of parallel experiments, two 2L perfusion reactors of the same size were respectively loaded with a microfiltration membrane with a nominal pore size of 1μm and a cell retention pad of competing products as perfusion filtration components. The protocol for this test is to stop the experiment when there is cell leakage, blockage of the retention pad, and cell viability drops below 90%.
During the 8-day perfusion cell culture experiment, the cell density in the bioreactor with Cellgard in, reaches nearly 120 M/ml. In the culture of Day 7-8, the TMP increased slightly and the cell viability decreased slightly, but no cell leakage was observed in the post-membrane harvest solution. Compared with the parallel control group, there was no significant difference in the cell viability during the same period, as shown in Figure 8.
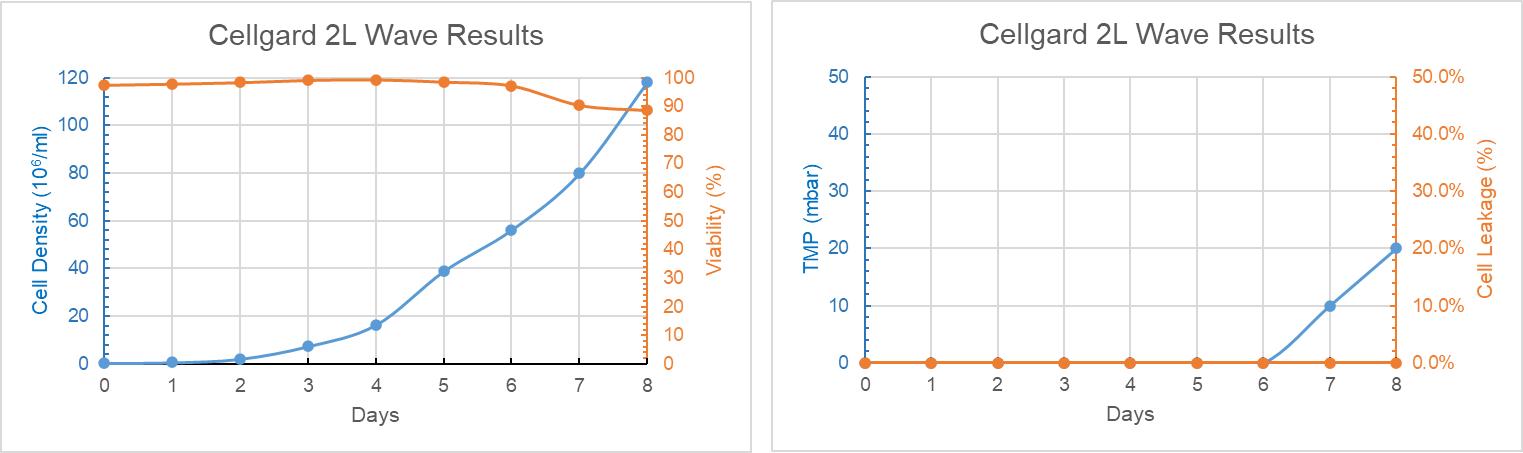 Figure 8: Cellgard 2L WAVE Trend Graph of Culture Days Versus Cell Density, Cell Viability, TMP and Cell Leakage
Figure 8: Cellgard 2L WAVE Trend Graph of Culture Days Versus Cell Density, Cell Viability, TMP and Cell Leakage
The bioreactors with MF membrane was plugged heavily at day 5, with ~30% of cell break-through downstream at the same time, and the cell viability was good, as shown in Figure 9.
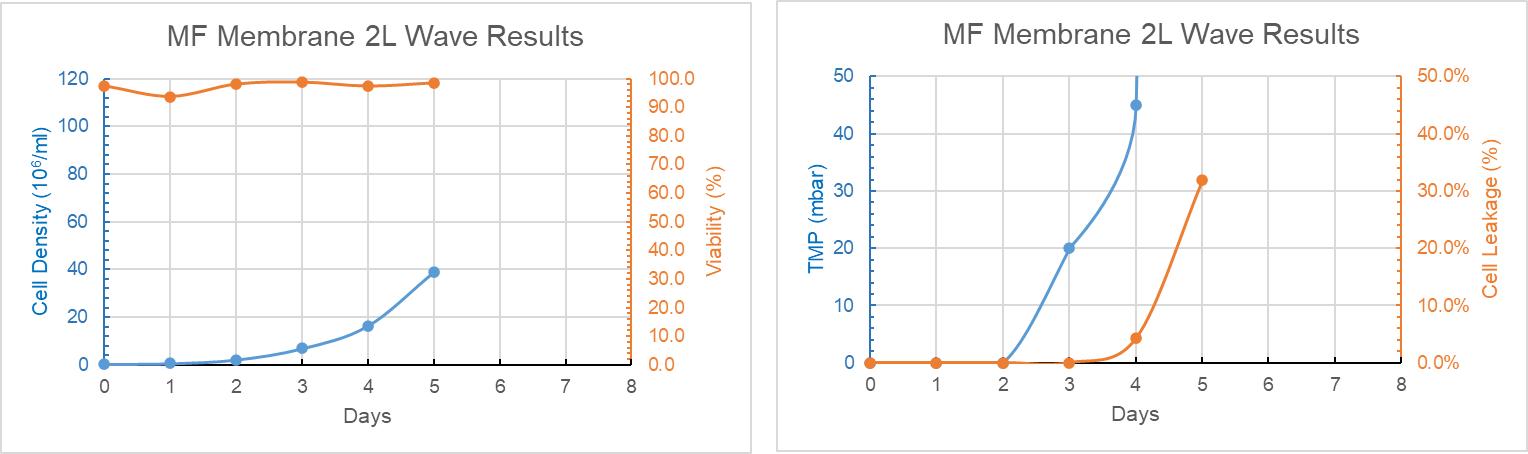 Figure 9: MF 2L WAVE Trend Graph of Culture Days Versus Cell Density, Cell Viability, TMP and Cell Leakage
Figure 9: MF 2L WAVE Trend Graph of Culture Days Versus Cell Density, Cell Viability, TMP and Cell Leakage
The bioreactors with competitor’s filter pad leaked at day 3, and leakage went up to 30% at day 5. The cell viability of the later two bioreactors were fairly good, as shown in Figure 10.
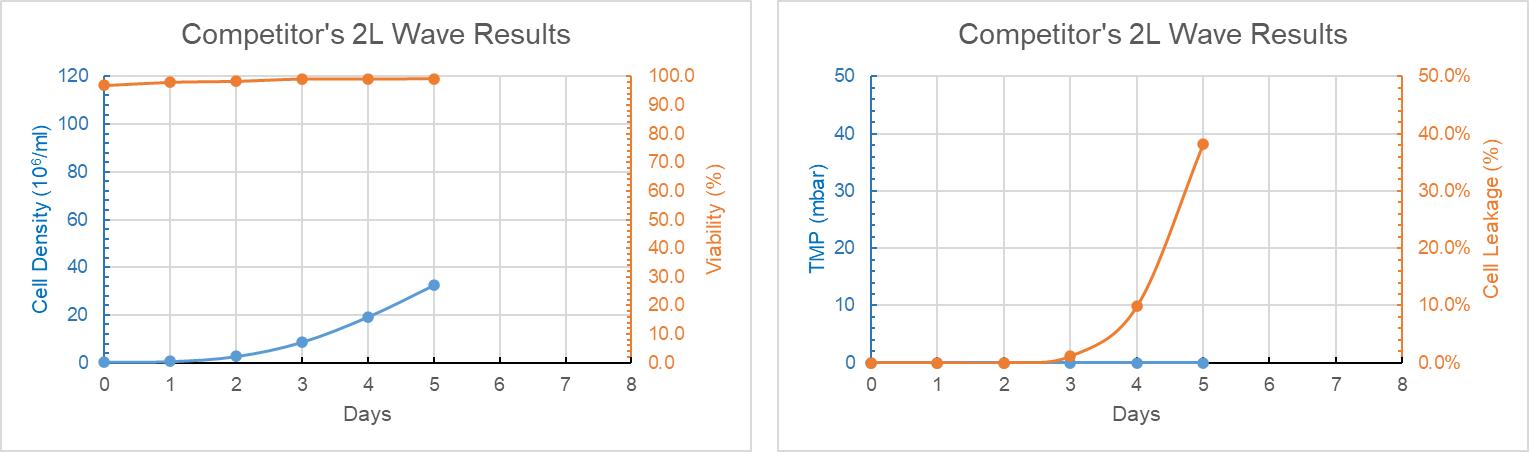 Figure 10: Competitor’s Filter Pad 2L WAVE Trend Graph of Culture Days Versus Cell Density, Cell Viability, TMP and Cell Leakage
Figure 10: Competitor’s Filter Pad 2L WAVE Trend Graph of Culture Days Versus Cell Density, Cell Viability, TMP and Cell Leakage
Experiment 2: 50L Wave Perfusion
The second set of tests focused on the performance of the Cellgard retention pad as a filtration component when cultured cells expressed proteins. The size of the Cellgard retention pad used is 30×50cm and the volume of the reactor is 50L. The preset goal of this culture experiment was to culture CHO cells to a cell concentration of 70 M/ml using perfusion bioreactors and maintain them for at least 5 days, while observing protein expression and protein yield in the harvest solution.
The inoculation cell density of CHO was 0.3 M/ml. After 3 days, the CHO cells passed into logarithmic growth phase smoothly. Cell density reached peak value of 84.7 M/ml at Day 10. The cell density was maintained at above 70 M/ml by culturing parameter adjustment. The cell viability was good during this period, as shown in Figure 11.
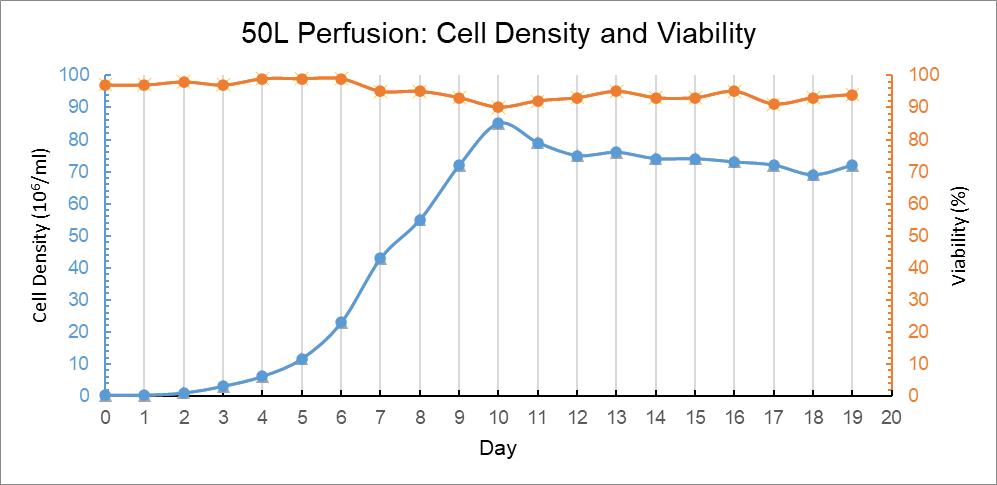 Figure 11: 50L Perfusion Bag Trend Graph of Culture Days Versus Cell Density and Cell Viability
Figure 11: 50L Perfusion Bag Trend Graph of Culture Days Versus Cell Density and Cell Viability
At the same time, the expression of cell protein also entered the corresponding peak. By measuring the protein concentration in the reactor and downstream of the retention pad, the protein yield at the harvest end was obtained. The difference in protein concentration could hardly be detected upstream and downstream of Cellgard cell retention pad, and the protein harvest rate was nearly 100%, as shown in Figure 12. This feature has a unique advantage compared with the devices that use microfiltration membrane and ultrafiltration membrane technology to realize the perfusion function.
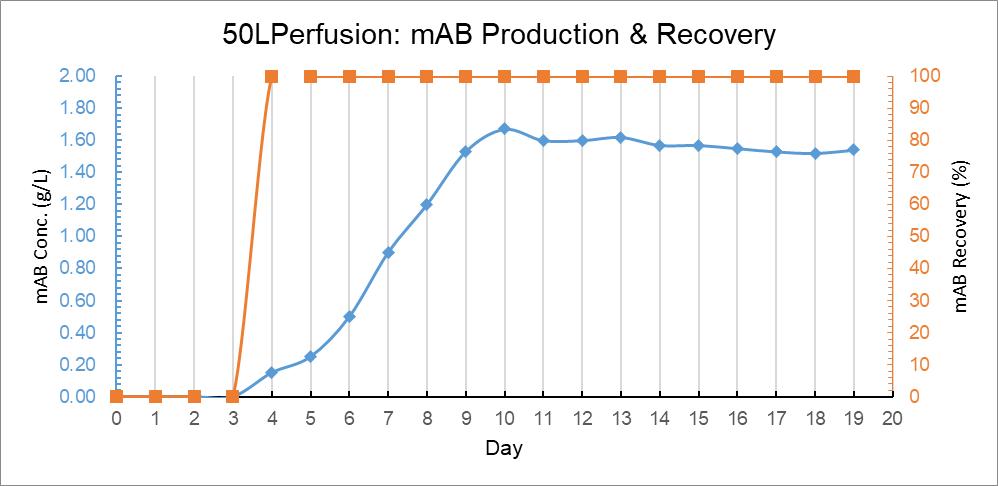 Figure 12: 50L Perfusion Bag Trend Graph of Culture Days Versus MAB Concentration and mAB Yield
Figure 12: 50L Perfusion Bag Trend Graph of Culture Days Versus MAB Concentration and mAB Yield
At Day 18, there were live cells detected downstream of Cellgard pad. The TMP of Cellgard reaches 50mBar at that time and it continued to grow as the perfusion culturing went on. So as the cell leakage did. The protein production rate and recovery seemed unaffected, as shown in Figure 13. The cell leakage was due to the increase of TMP, which caused by pore plug of Cellgard pad. We recommend our customers to monitor the TMP to decide when to stop.
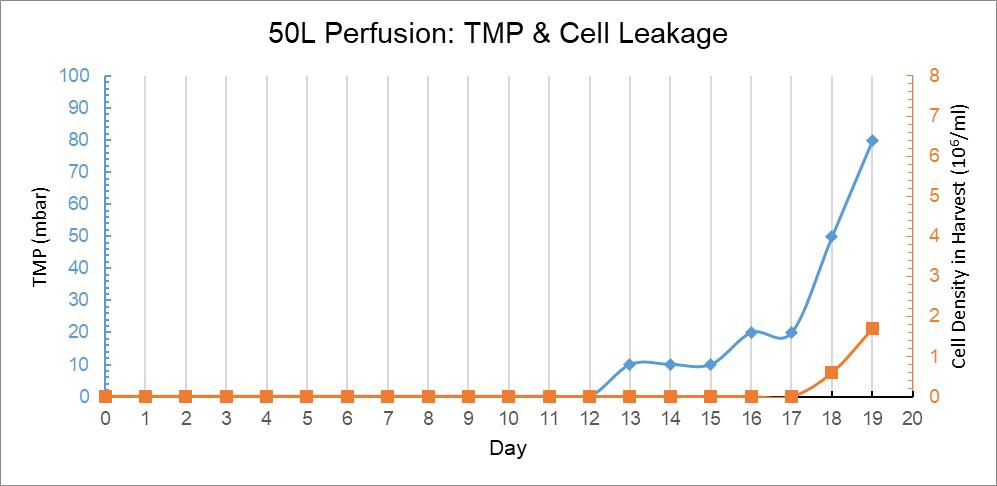 Figure 13: 50L Perfusion Bag Trend Graph of Culture Days Versus TMP and Harvest Fluid Cell Density
Figure 13: 50L Perfusion Bag Trend Graph of Culture Days Versus TMP and Harvest Fluid Cell Density
At this point, under two different cultivation process conditions, the Cellgard retention pad achieved the goals of high cell culture density, high retention rate, and long service life, and the cultivation situation met the experimental expectations. Cellgard products can provide strong support for high-density cell culture in single-use perfusion bioreactors. It should be noted that different cultivation techniques used by users may result in different cultivation results. The above two sets of experimental data are for reference only and cannot be considered as standard results.
Advantages of Membrane Solutions Cellgard Cell Retention Pad
- Using the depth filtration mechanism, higher density cell culture can be achieved, and the anti-clogging ability is strong
- Higher mechanical strength to avoid leakage caused by mechanical damage during use
- Special pore size design ensures almost no retention of target products such as proteins under the premise of avoiding cell leakage
- Cellgard cell retention pad products are now open for trial, welcome new and old customers to inquire. Product size can be customized according to user needs. Please consult the MS sales engineer around you for details.
References
【1】Clincke M F, Mölleryd C, Samani P K, et al. Very high density of Chinese hamster ovary cells in perfusion by alternating tangential flow or tangential flow filtration in WAVE bioreactor™—part II: Applications for antibody production and cryopreservation[J]. Biotechnology progress, 2013, 29(3): 768-777.
【2】Wright B, Bruninghaus M, Vrabel M, et al. A novel seed-train process: using high-density cell banking, a disposable bioreactor, and perfusion technologies[J]. Bioprocess Int, 2015, 13(3): 16-25.
